The vapor pressure of pure h 2 o at 52 0 c is 102 1 torr the density of h 2 o at 52 0 c is 0 987 g ml. What is the change in vapor pressure when 52 9 g of cucl 2 is added to 800 ml of h 2 o at 52 0 c. We can calculate the vapor pressure of the solvent in a solution p solv note there is no superscript o in this symbol in two ways.

Vapor pressure of a solution. We calculate the change in vapor pressure of the solvent p solv using the following equation. The vapor pressure of the pure solvent p o solv.

In order to solve for raoult s law the mole fraction of water must be obtained. The vapor pressure of pure water at 25 c is 23 8 torr answer. What is the vapor pressure of a solution at 25 c containing 3 5 moles of glucose in 10 0 moles of water.

Vapor pressure formula questions. K p w. H 2 l h 2 g the equilibrium constant for this reaction the vaporization reaction is.

The transition from liquid water to steam for example may be written as. A phase change may be written as a chemical reaction. Phase changes vapor pressure concepts.

It relates to the tendency of particles to. See spelling differences or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases solid or liquid at a given temperature in a closed system the equilibrium vapor pressure is an indication of a liquid s evaporation rate. Vapor pressure or vapour pressure in british english.

This is the formula you ll use to solve the most common sorts of vapor pressure problems you ll find in physics and chemistry classes.
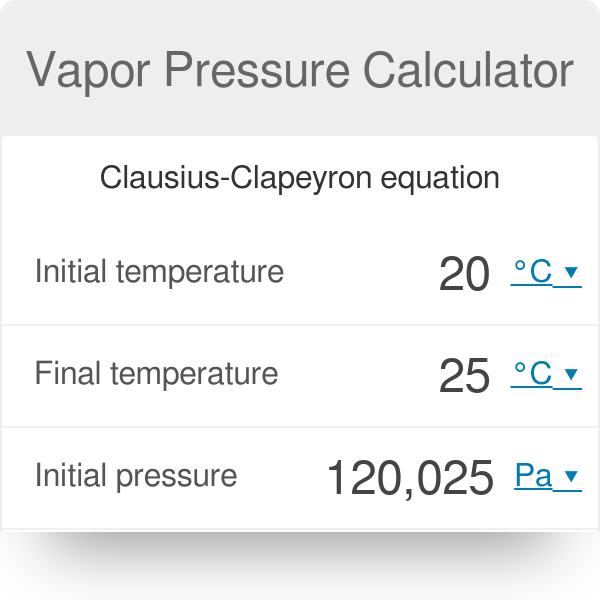
Change in vapor pressure formula. Write the clausius clapeyron equation. The formula used for calculating vapor pressure given a change in the vapor pressure over time is known as the clausius clapeyron equation named for physicists rudolf clausius and benoît paul émile clapeyron.

The formula used for calculating vapor pressure given a change in the vapor pressure over time is known as the clausius clapeyron equation named for physicists rudolf clausius and benoît paul émile clapeyron. Write the clausius clapeyron equation.