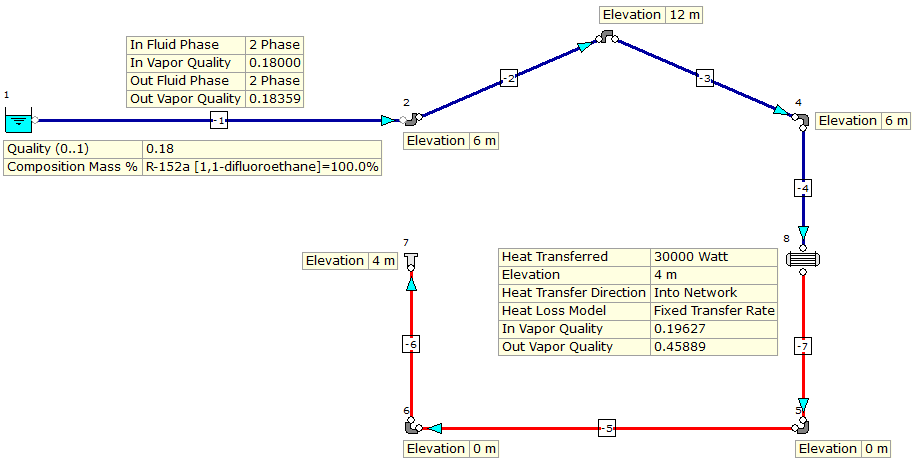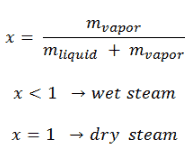
I do have a table containing all necessary data such as temperature specific volume internal energy enthalpy and entropy at the afore mention pressure states. Homework equations no clue. What is the quality x of the steam after expansion.

Saturated steam with a pressure of 30 bars is isentropically expanded to a pressure of 1 bar. The mass friction of a vapor in a liquid vapor mixture in a two phase liquid vapor regoin is called vapor quality also called dryness fraction. It will not condense when small amounts of heat is removed.
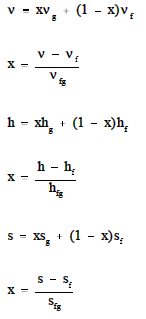
Superheated vapor is when vapor has absorbed more heat than is needed to vaporize. For example if an amount q of heat is applied to a mass of liquid m at saturation temperature then the mass of vapor generated is m g q δh lg where δh lg is the latent heat of vaporization. In thermal equilibrium the quality of a two phase mixture is directly related to heat input and is sometimes called the thermodynamic quality.

I need the formula or the direction in which i can think to figure out the question. How do i calculate the heat flux that the water is subjected to during flowing through the channel. I also know that the water is coming out of the channel at a vapor quality of 1.

And i know the inlet properties of water. I know the mass flow rate of water. Vapour quality is an intensive property which can be used in conjunction with other independent intensive properties to specify the thermodynamic state of the working fluid of a thermodynamic system.
1 in other words saturated vapour has a quality of 100 and saturated liquid has a quality of 0. In thermodynamics vapour quality is the mass fraction in a saturated mixture that is vapour. Calculate properties of combustion gases.
Calculate online thermodynamic and transport properties of water and steam based on industrial iapws if97 or scientific iapws 95 formulation.
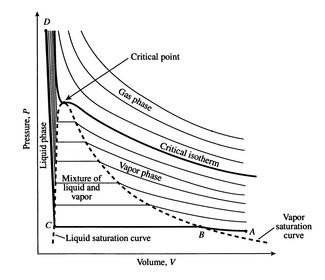
Vapor quality formula. Vapor quality is an important quantity during the adiabatic expansion step in various thermodynamic cycles like organic rankine cycle rankine cycle etc. Working fluids can be classified by using the appearance of droplets in the vapour during the expansion step. Wet steam is characterized by the vapor quality which ranges from zero to unity open interval 0 1 when the vapor quality is equal to 0 it is referred to as the saturated liquid state single phase. On the other hand when the vapor quality is equal to 1 it is referred to as the saturated vapor state or dry steam single phase.
Wet steam is characterized by the vapor quality which ranges from zero to unity open interval 0 1 when the vapor quality is equal to 0 it is referred to as the saturated liquid state single phase. On the other hand when the vapor quality is equal to 1 it is referred to as the saturated vapor state or dry steam single phase. State e was all saturated vapor all of the mass in the system was vapor so its quality would be one. All states intermediate between c and e would have a quality between zero and one.
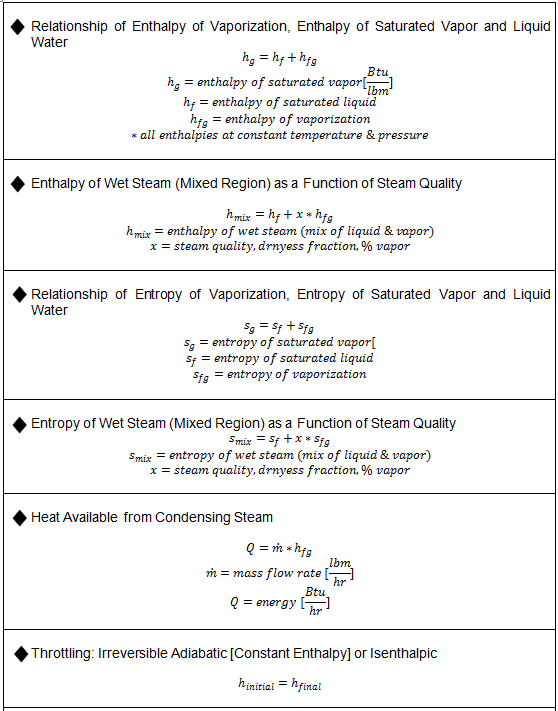
Substituting equation 7 into 6 yields. 8 v x v g 1 x v f. Which provides a relation between specific volume v and quality x in the two phase region.
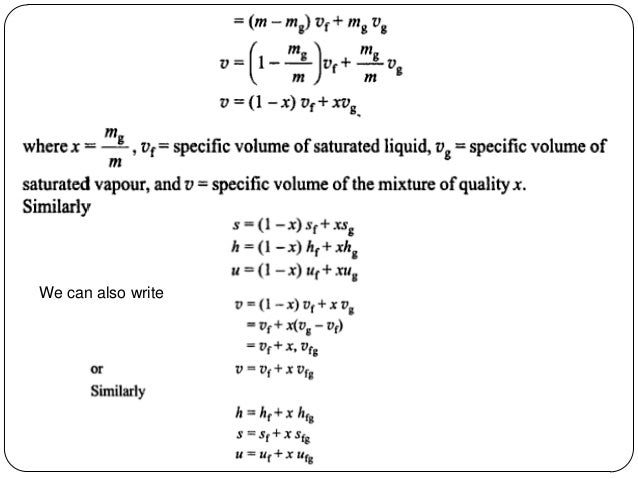
Which provides a relation between specific volume v and quality x in the two phase region. 8 v x v g 1 x v f. Substituting equation 7 into 6 yields.
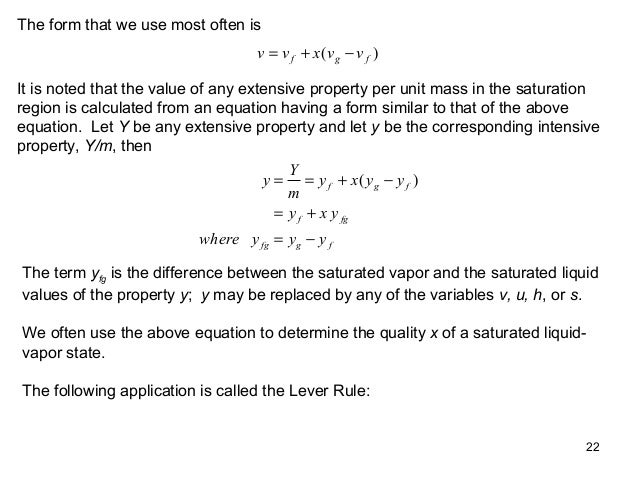
All states intermediate between c and e would have a quality between zero and one. State e was all saturated vapor all of the mass in the system was vapor so its quality would be one. On the other hand when the vapor quality is equal to 1 it is referred to as the saturated vapor state or dry steam single phase.

Wet steam is characterized by the vapor quality which ranges from zero to unity open interval 0 1 when the vapor quality is equal to 0 it is referred to as the saturated liquid state single phase. On the other hand when the vapor quality is equal to 1 it is referred to as the saturated vapor state or dry steam single phase. Wet steam is characterized by the vapor quality which ranges from zero to unity open interval 0 1 when the vapor quality is equal to 0 it is referred to as the saturated liquid state single phase.
Working fluids can be classified by using the appearance of droplets in the vapour during the expansion step. Vapor quality is an important quantity during the adiabatic expansion step in various thermodynamic cycles like organic rankine cycle rankine cycle etc.