
This basically means that for an ideal mixture the number of moles of each component of the mixture present in the. 0 463 your tool of choice here will be raoult s law which states that at a given temperature the vapor pressure of a volatile component in an ideal mixture is equal to the mole fraction of the component in the mixture multiplied by the vapor pressure of the pure component. But if the liquid is kept in a closed container the gaseous molecules will not escape in the air but will remain above the liquid.

Vapor pressure formula whenever the liquid evaporates the gaseous molecules formed will escape in the air. H 2 o 0 741. In order to solve for raoult s law the mole fraction of water must be obtained.

The vapor pressure of pure water at 25 c is 23 8 torr answer. What is the vapor pressure of a solution at 25 c containing 3 5 moles of glucose in 10 0 moles of water. Vapor pressure formula questions.

The value of the quality ranges from zero to unity. The mass fraction of the vapor in a two phase liquid vapor region is called the vapor quality or dryness fraction x and it is given by following formula. The value of the quality ranges from zero to unity.
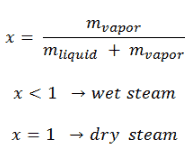
The mass fraction of the vapor in a two phase liquid vapor region is called the vapor quality or dryness fraction x and it is given by following formula. In consequence the relative lowering of vapour pressure of a dilute solution. It states that the partial pressure of each component of an ideal mixture of liquids is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture.

Raoult s law ˈ r ɑː uː l z law is a law of thermodynamics established by french chemist françois marie raoult in 1887. The higher temperatures of a metallic surface can propagate an increase in the vapor fraction of the metallic environment because more vapor is formed at higher temperatures. Vapor fraction refers to the ratio quantity of the gaseous component present in a mixture of two or more physical states.

Vapour quality is an intensive property which can be used in conjunction with other independent intensive properties to specify the thermodynamic state of the working fluid of a thermodynamic system.
Vapor fraction formula. In thermodynamics vapour quality is the mass fraction in a saturated mixture that is vapour. In other words saturated vapour has a quality of 100 and saturated liquid has a quality of 0.
In other words saturated vapour has a quality of 100 and saturated liquid has a quality of 0. In thermodynamics vapour quality is the mass fraction in a saturated mixture that is vapour.











